Global Multiple Myeloma Diagnostic Market Size, Share & Trends Analysis Report, Forecast Period, 2023-2031
Report ID: MS-2096 | Healthcare and Pharma | Last updated: Nov, 2024 | Formats*:
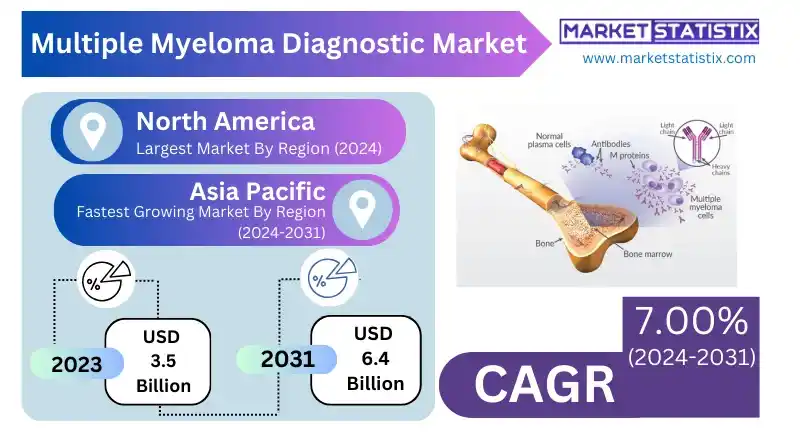
Multiple Myeloma Diagnostic Report Highlights
| Report Metrics | Details |
|---|---|
| Forecast period | 2019-2031 |
| Base Year Of Estimation | 2023 |
| Growth Rate | CAGR of 7.00% |
| Forecast Value (2031) | USD 6.4 Billion |
| By Product Type | Molecular Diagnostics, Cytogenetics, Immunoassays, Flow Cytometry, Others |
| Key Market Players |
|
| By Region |
|
Multiple Myeloma Diagnostic Market Trends
The multiple myeloma diagnostic market has seen some very key trends influenced by innovations in technology and greater understanding of the disease. One such is the increased use of molecular diagnostic techniques, including NGS and liquid biopsy, to detect the condition at a more advanced and earlier stage of multiple myeloma with higher accuracy. These innovations allow better risk stratification and personalized treatment planning that leads to improved outcomes in these patients. The implementation of AI and machine learning in diagnostic devices further optimizes the disease detection and monitoring procedures through the analysis of sophisticated data flows from blood tests, imaging techniques, and genetic profiling. One of the most observed trends here is the increasing demand for combination testing and companion diagnostics in order to tailor therapies based on specific biomarkers or mutations. A pattern emerging in diagnostic tests among the targeted and individualized treatment options for multiple myeloma creates this growing demand for the multiple myeloma diagnostic market worldwide.Multiple Myeloma Diagnostic Market Leading Players
The key players profiled in the report are Illumina Inc., Thermo Fisher Scientific Inc., Merck & Co. Inc., Takeda Pharmaceutical Company Limited, GlaxoSmithKline plc, Amgen Inc., Abbott Laboratories, Celgene Corporation, Bristol-Myers Squibb Company, Agilent Technologies, Inc., Qiagen N.V., PerkinElmer Inc., Siemens Healthineers AG, Bio-Rad Laboratories Inc., Novartis AG, Pfizer Inc., Sanofi S.A., F. Hoffmann-La Roche Ltd, Myriad Genetics Inc., Janssen Pharmaceuticals, Inc.Growth Accelerators
The market drivers for the diagnostic market for multiple myeloma are an increase in the incidence rate of the disease globally and an increasing demand for an early diagnosis. Advanced age is a major risk factor for the increasing prevalence of multiple myeloma, a type of blood cancer, that makes them require a proper diagnosis and treatment with more advanced and effective diagnostic tools. The urge to seek early diagnosis plays a key role in determining the patient outcome. For the development and adoption of such accurate diagnostic techniques like genetic testing, biomarkers, and imaging technology, it becomes even more important. Since the awareness grows, so does the market growth. The multiple myeloma diagnostic market is driven further by advanced diagnostic technologies alongside increased health care investments. For instance, developments in next-generation sequencing, liquid biopsy, and more advanced imaging are enhancing the precision and speed at which care is diagnosed, which translates into better treatment planning. Better funds spent in cancer research, along with collaborations between diagnostic firms and healthcare providers, expand this market further. The health care system may identify more cases of multiple myeloma with increased diagnostic accuracy and non-invasive options, thereby enhancing patient care and the management of the disease itself.Multiple Myeloma Diagnostic Market Segmentation analysis
The Global Multiple Myeloma Diagnostic is segmented by Type, and Region. By Type, the market is divided into Distributed Molecular Diagnostics, Cytogenetics, Immunoassays, Flow Cytometry, Others . Geographically, the market is assessed across key Regions like North America(United States, Canada, Mexico), South America(Brazil, Argentina, Chile, Rest of South America), Europe(Germany, France, Italy, United Kingdom, Benelux, Nordics, Rest of Europe), Asia Pacific(China, Japan, India, South Korea, Australia, Southeast Asia, Rest of Asia-Pacific), MEA(Middle East, Africa) and others, each presenting distinct growth opportunities and challenges influenced by the regions.Competitive Landscape
Of the competitive landscape, there are leading players like Abbott Laboratories, Roche Diagnostics, and Siemens Healthineers actively working on developing innovative solutions and commercializing them for the diagnosis of multiple myeloma. These companies have been focusing on strategic collaborations and partnerships to enhance their product offerings and expand their geographic reach. This is supplemented further by the emergence of new players, introducing novel diagnostic equipment that rely on artificial intelligence and machine learning in their detection ability, thereby raising their accuracy levels. Further research, particularly on the genetic factors of multiple myeloma, will drive diagnosis intensity further, with competition between well-established market participants and new entrants also increasing in intensity.Challenges In Multiple Myeloma Diagnostic Market
The challenges in diagnosing myeloma come from the very process of diagnosis, which is complicated in the case of this disease. In the early stages, most cases of multiple myeloma remain undiagnosed because, being mainly nonspecific, their signs and symptoms do not trigger medical attention. Such diagnostic instruments should have high specificity to differentiate multiple myeloma from other diseases that mimic it, such as other blood cancers or bone diseases. Of course, expensive advanced techniques of diagnosis, like genetic tests and specialty-specific imagery, may limit access to low-resource settings. One such concern in this regard is the lack of uniform protocols for diagnosis among regions, thereby causing inconsistency and delay in diagnosis. The presence of variation in diagnostic practices makes it harder to adopt advanced technologies, and such factors add to the inefficiency of diagnostic processes. Overall, it creates impediments to the widespread adoption of advanced diagnostic solutions for early and accurate detection of multiple myeloma.Risks & Prospects in Multiple Myeloma Diagnostic Market
The marketplace for the diagnosis of multiple myeloma is a significant opportunity, as it has a rising incidence, higher awareness, and advanced diagnostic technology. Age across the globe is increasing, accounting for a significant proportion of the incidence of multiple myeloma, thus requiring early detection and accurate diagnosis of the disease. Liquid biopsy, next-generation sequencing, and advanced imaging techniques promise great opportunities for companies to develop tests that are more efficient and less invasive. Advancements could improve early-stage detection, thereby allowing better patient outcomes and the growth of the diagnostic market. The diagnostic market for multiple myeloma offers more opportunities because emphasis on the field of personalized medicine and precision diagnostics continues to rise. With increased use of tailored treatments, development of accurate biomarkers, and platforms for diagnosis of disease progression and response and treatment to minimal residual disease have to be built. It has become a significant market opportunity for companies in diagnostics to help develop and commercialize innovative solutions that serve as support towards clinicians in delivering personalized care to patients with multiple myeloma and subsequently improving not just the treatment effectivity but the quality of life of the patients.Key Target Audience
The key target audience of the multiple myeloma diagnostic market is hospitals, diagnostic laboratories, and oncologists, as they play a very critical role in the early diagnosis and detection of multiple myeloma. They use advanced diagnostic tools and technologies such as blood tests, imaging, and bone marrow biopsy to diagnose the presence of multiple myeloma in patients. The increased incidence of cancer and the advancement of new diagnostic technologies keep the attention of healthcare providers concentrated on accurate and timely diagnosis to enhance patient outcomes.,, Apart from the healthcare providers, pharmaceutical companies and research institutions are important players in the market for diagnosing multiple myeloma. These entities contribute to discovering new tests, biomarkers, and technologies that allow enhancements in the detection and monitoring of the disease. With the research and development of personalized medicine combined with the ever-escalating demand to make better diagnostic solutions, these groups are at the core of innovations within the market and focus on improving such accuracies and speed in diagnosing multiple myeloma for better planning.Merger and acquisition
In recent mergers and acquisitions in the multiple myeloma diagnostics market, the leading companies are set to consolidate their efforts in order to provide better solutions and increase services. The most recent acquisition occurred in October 2022, with Thermo Fisher Scientific purchasing Binding Site Group, the market leader in the protein diagnosis of multiple myeloma. This acquisition is being done to enhance the diagnostic capacities of Thermo Fisher, primarily within the realm of oncology, so that complete diagnostic solutions may be delivered to complement the therapies applied to the patients suffering from myeloma. The strategic steps taken within this industry suggest how much advanced diagnostic technologies are being implemented to improve patient care and simplify tests. Apart from Thermo Fisher's deal, several others have been fairly significant in shaping the multiple myeloma diagnostics market. For instance, SD Biosensor, in collaboration with SJL Partners, acquired Meridian Bioscience for a sum of $1.5 billion to enhance its innovative portfolio of diagnostic products. Roche, for its part, has made a buyout of GenMark for $1.8 billion, expanding the company's molecular diagnosis capabilities, including tests relevant to multiple myeloma.- 1.1 Report description
- 1.2 Key market segments
- 1.3 Key benefits to the stakeholders
2: Executive Summary
- 2.1 Multiple Myeloma Diagnostic- Snapshot
- 2.2 Multiple Myeloma Diagnostic- Segment Snapshot
- 2.3 Multiple Myeloma Diagnostic- Competitive Landscape Snapshot
3: Market Overview
- 3.1 Market definition and scope
- 3.2 Key findings
- 3.2.1 Top impacting factors
- 3.2.2 Top investment pockets
- 3.3 Porter’s five forces analysis
- 3.3.1 Low bargaining power of suppliers
- 3.3.2 Low threat of new entrants
- 3.3.3 Low threat of substitutes
- 3.3.4 Low intensity of rivalry
- 3.3.5 Low bargaining power of buyers
- 3.4 Market dynamics
- 3.4.1 Drivers
- 3.4.2 Restraints
- 3.4.3 Opportunities
4: Multiple Myeloma Diagnostic Market by Type
- 4.1 Overview
- 4.1.1 Market size and forecast
- 4.2 Immunoassays
- 4.2.1 Key market trends, factors driving growth, and opportunities
- 4.2.2 Market size and forecast, by region
- 4.2.3 Market share analysis by country
- 4.3 Flow Cytometry
- 4.3.1 Key market trends, factors driving growth, and opportunities
- 4.3.2 Market size and forecast, by region
- 4.3.3 Market share analysis by country
- 4.4 Cytogenetics
- 4.4.1 Key market trends, factors driving growth, and opportunities
- 4.4.2 Market size and forecast, by region
- 4.4.3 Market share analysis by country
- 4.5 Molecular Diagnostics
- 4.5.1 Key market trends, factors driving growth, and opportunities
- 4.5.2 Market size and forecast, by region
- 4.5.3 Market share analysis by country
- 4.6 Others
- 4.6.1 Key market trends, factors driving growth, and opportunities
- 4.6.2 Market size and forecast, by region
- 4.6.3 Market share analysis by country
5: Multiple Myeloma Diagnostic Market by End-User
- 5.1 Overview
- 5.1.1 Market size and forecast
- 5.2 Hospitals
- 5.2.1 Key market trends, factors driving growth, and opportunities
- 5.2.2 Market size and forecast, by region
- 5.2.3 Market share analysis by country
- 5.3 Diagnostic Laboratories
- 5.3.1 Key market trends, factors driving growth, and opportunities
- 5.3.2 Market size and forecast, by region
- 5.3.3 Market share analysis by country
- 5.4 Academic and Research Institutes
- 5.4.1 Key market trends, factors driving growth, and opportunities
- 5.4.2 Market size and forecast, by region
- 5.4.3 Market share analysis by country
- 5.5 Others
- 5.5.1 Key market trends, factors driving growth, and opportunities
- 5.5.2 Market size and forecast, by region
- 5.5.3 Market share analysis by country
6: Multiple Myeloma Diagnostic Market by Region
- 6.1 Overview
- 6.1.1 Market size and forecast By Region
- 6.2 North America
- 6.2.1 Key trends and opportunities
- 6.2.2 Market size and forecast, by Type
- 6.2.3 Market size and forecast, by Application
- 6.2.4 Market size and forecast, by country
- 6.2.4.1 United States
- 6.2.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.1.2 Market size and forecast, by Type
- 6.2.4.1.3 Market size and forecast, by Application
- 6.2.4.2 Canada
- 6.2.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.2.2 Market size and forecast, by Type
- 6.2.4.2.3 Market size and forecast, by Application
- 6.2.4.3 Mexico
- 6.2.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.2.4.3.2 Market size and forecast, by Type
- 6.2.4.3.3 Market size and forecast, by Application
- 6.2.4.1 United States
- 6.3 South America
- 6.3.1 Key trends and opportunities
- 6.3.2 Market size and forecast, by Type
- 6.3.3 Market size and forecast, by Application
- 6.3.4 Market size and forecast, by country
- 6.3.4.1 Brazil
- 6.3.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.1.2 Market size and forecast, by Type
- 6.3.4.1.3 Market size and forecast, by Application
- 6.3.4.2 Argentina
- 6.3.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.2.2 Market size and forecast, by Type
- 6.3.4.2.3 Market size and forecast, by Application
- 6.3.4.3 Chile
- 6.3.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.3.2 Market size and forecast, by Type
- 6.3.4.3.3 Market size and forecast, by Application
- 6.3.4.4 Rest of South America
- 6.3.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.3.4.4.2 Market size and forecast, by Type
- 6.3.4.4.3 Market size and forecast, by Application
- 6.3.4.1 Brazil
- 6.4 Europe
- 6.4.1 Key trends and opportunities
- 6.4.2 Market size and forecast, by Type
- 6.4.3 Market size and forecast, by Application
- 6.4.4 Market size and forecast, by country
- 6.4.4.1 Germany
- 6.4.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.1.2 Market size and forecast, by Type
- 6.4.4.1.3 Market size and forecast, by Application
- 6.4.4.2 France
- 6.4.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.2.2 Market size and forecast, by Type
- 6.4.4.2.3 Market size and forecast, by Application
- 6.4.4.3 Italy
- 6.4.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.3.2 Market size and forecast, by Type
- 6.4.4.3.3 Market size and forecast, by Application
- 6.4.4.4 United Kingdom
- 6.4.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.4.2 Market size and forecast, by Type
- 6.4.4.4.3 Market size and forecast, by Application
- 6.4.4.5 Benelux
- 6.4.4.5.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.5.2 Market size and forecast, by Type
- 6.4.4.5.3 Market size and forecast, by Application
- 6.4.4.6 Nordics
- 6.4.4.6.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.6.2 Market size and forecast, by Type
- 6.4.4.6.3 Market size and forecast, by Application
- 6.4.4.7 Rest of Europe
- 6.4.4.7.1 Key market trends, factors driving growth, and opportunities
- 6.4.4.7.2 Market size and forecast, by Type
- 6.4.4.7.3 Market size and forecast, by Application
- 6.4.4.1 Germany
- 6.5 Asia Pacific
- 6.5.1 Key trends and opportunities
- 6.5.2 Market size and forecast, by Type
- 6.5.3 Market size and forecast, by Application
- 6.5.4 Market size and forecast, by country
- 6.5.4.1 China
- 6.5.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.1.2 Market size and forecast, by Type
- 6.5.4.1.3 Market size and forecast, by Application
- 6.5.4.2 Japan
- 6.5.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.2.2 Market size and forecast, by Type
- 6.5.4.2.3 Market size and forecast, by Application
- 6.5.4.3 India
- 6.5.4.3.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.3.2 Market size and forecast, by Type
- 6.5.4.3.3 Market size and forecast, by Application
- 6.5.4.4 South Korea
- 6.5.4.4.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.4.2 Market size and forecast, by Type
- 6.5.4.4.3 Market size and forecast, by Application
- 6.5.4.5 Australia
- 6.5.4.5.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.5.2 Market size and forecast, by Type
- 6.5.4.5.3 Market size and forecast, by Application
- 6.5.4.6 Southeast Asia
- 6.5.4.6.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.6.2 Market size and forecast, by Type
- 6.5.4.6.3 Market size and forecast, by Application
- 6.5.4.7 Rest of Asia-Pacific
- 6.5.4.7.1 Key market trends, factors driving growth, and opportunities
- 6.5.4.7.2 Market size and forecast, by Type
- 6.5.4.7.3 Market size and forecast, by Application
- 6.5.4.1 China
- 6.6 MEA
- 6.6.1 Key trends and opportunities
- 6.6.2 Market size and forecast, by Type
- 6.6.3 Market size and forecast, by Application
- 6.6.4 Market size and forecast, by country
- 6.6.4.1 Middle East
- 6.6.4.1.1 Key market trends, factors driving growth, and opportunities
- 6.6.4.1.2 Market size and forecast, by Type
- 6.6.4.1.3 Market size and forecast, by Application
- 6.6.4.2 Africa
- 6.6.4.2.1 Key market trends, factors driving growth, and opportunities
- 6.6.4.2.2 Market size and forecast, by Type
- 6.6.4.2.3 Market size and forecast, by Application
- 6.6.4.1 Middle East
- 7.1 Overview
- 7.2 Key Winning Strategies
- 7.3 Top 10 Players: Product Mapping
- 7.4 Competitive Analysis Dashboard
- 7.5 Market Competition Heatmap
- 7.6 Leading Player Positions, 2022
8: Company Profiles
- 8.1 Sanofi S.A.
- 8.1.1 Company Overview
- 8.1.2 Key Executives
- 8.1.3 Company snapshot
- 8.1.4 Active Business Divisions
- 8.1.5 Product portfolio
- 8.1.6 Business performance
- 8.1.7 Major Strategic Initiatives and Developments
- 8.2 GlaxoSmithKline plc
- 8.2.1 Company Overview
- 8.2.2 Key Executives
- 8.2.3 Company snapshot
- 8.2.4 Active Business Divisions
- 8.2.5 Product portfolio
- 8.2.6 Business performance
- 8.2.7 Major Strategic Initiatives and Developments
- 8.3 Novartis AG
- 8.3.1 Company Overview
- 8.3.2 Key Executives
- 8.3.3 Company snapshot
- 8.3.4 Active Business Divisions
- 8.3.5 Product portfolio
- 8.3.6 Business performance
- 8.3.7 Major Strategic Initiatives and Developments
- 8.4 Siemens Healthineers AG
- 8.4.1 Company Overview
- 8.4.2 Key Executives
- 8.4.3 Company snapshot
- 8.4.4 Active Business Divisions
- 8.4.5 Product portfolio
- 8.4.6 Business performance
- 8.4.7 Major Strategic Initiatives and Developments
- 8.5 Merck & Co. Inc.
- 8.5.1 Company Overview
- 8.5.2 Key Executives
- 8.5.3 Company snapshot
- 8.5.4 Active Business Divisions
- 8.5.5 Product portfolio
- 8.5.6 Business performance
- 8.5.7 Major Strategic Initiatives and Developments
- 8.6 Agilent Technologies
- 8.6.1 Company Overview
- 8.6.2 Key Executives
- 8.6.3 Company snapshot
- 8.6.4 Active Business Divisions
- 8.6.5 Product portfolio
- 8.6.6 Business performance
- 8.6.7 Major Strategic Initiatives and Developments
- 8.7 Inc.
- 8.7.1 Company Overview
- 8.7.2 Key Executives
- 8.7.3 Company snapshot
- 8.7.4 Active Business Divisions
- 8.7.5 Product portfolio
- 8.7.6 Business performance
- 8.7.7 Major Strategic Initiatives and Developments
- 8.8 Bristol-Myers Squibb Company
- 8.8.1 Company Overview
- 8.8.2 Key Executives
- 8.8.3 Company snapshot
- 8.8.4 Active Business Divisions
- 8.8.5 Product portfolio
- 8.8.6 Business performance
- 8.8.7 Major Strategic Initiatives and Developments
- 8.9 Amgen Inc.
- 8.9.1 Company Overview
- 8.9.2 Key Executives
- 8.9.3 Company snapshot
- 8.9.4 Active Business Divisions
- 8.9.5 Product portfolio
- 8.9.6 Business performance
- 8.9.7 Major Strategic Initiatives and Developments
- 8.10 Qiagen N.V.
- 8.10.1 Company Overview
- 8.10.2 Key Executives
- 8.10.3 Company snapshot
- 8.10.4 Active Business Divisions
- 8.10.5 Product portfolio
- 8.10.6 Business performance
- 8.10.7 Major Strategic Initiatives and Developments
- 8.11 Illumina Inc.
- 8.11.1 Company Overview
- 8.11.2 Key Executives
- 8.11.3 Company snapshot
- 8.11.4 Active Business Divisions
- 8.11.5 Product portfolio
- 8.11.6 Business performance
- 8.11.7 Major Strategic Initiatives and Developments
- 8.12 Thermo Fisher Scientific Inc.
- 8.12.1 Company Overview
- 8.12.2 Key Executives
- 8.12.3 Company snapshot
- 8.12.4 Active Business Divisions
- 8.12.5 Product portfolio
- 8.12.6 Business performance
- 8.12.7 Major Strategic Initiatives and Developments
- 8.13 Takeda Pharmaceutical Company Limited
- 8.13.1 Company Overview
- 8.13.2 Key Executives
- 8.13.3 Company snapshot
- 8.13.4 Active Business Divisions
- 8.13.5 Product portfolio
- 8.13.6 Business performance
- 8.13.7 Major Strategic Initiatives and Developments
- 8.14 Pfizer Inc.
- 8.14.1 Company Overview
- 8.14.2 Key Executives
- 8.14.3 Company snapshot
- 8.14.4 Active Business Divisions
- 8.14.5 Product portfolio
- 8.14.6 Business performance
- 8.14.7 Major Strategic Initiatives and Developments
- 8.15 Celgene Corporation
- 8.15.1 Company Overview
- 8.15.2 Key Executives
- 8.15.3 Company snapshot
- 8.15.4 Active Business Divisions
- 8.15.5 Product portfolio
- 8.15.6 Business performance
- 8.15.7 Major Strategic Initiatives and Developments
- 8.16 Abbott Laboratories
- 8.16.1 Company Overview
- 8.16.2 Key Executives
- 8.16.3 Company snapshot
- 8.16.4 Active Business Divisions
- 8.16.5 Product portfolio
- 8.16.6 Business performance
- 8.16.7 Major Strategic Initiatives and Developments
- 8.17 Myriad Genetics Inc.
- 8.17.1 Company Overview
- 8.17.2 Key Executives
- 8.17.3 Company snapshot
- 8.17.4 Active Business Divisions
- 8.17.5 Product portfolio
- 8.17.6 Business performance
- 8.17.7 Major Strategic Initiatives and Developments
- 8.18 F. Hoffmann-La Roche Ltd
- 8.18.1 Company Overview
- 8.18.2 Key Executives
- 8.18.3 Company snapshot
- 8.18.4 Active Business Divisions
- 8.18.5 Product portfolio
- 8.18.6 Business performance
- 8.18.7 Major Strategic Initiatives and Developments
- 8.19 Bio-Rad Laboratories Inc.
- 8.19.1 Company Overview
- 8.19.2 Key Executives
- 8.19.3 Company snapshot
- 8.19.4 Active Business Divisions
- 8.19.5 Product portfolio
- 8.19.6 Business performance
- 8.19.7 Major Strategic Initiatives and Developments
- 8.20 PerkinElmer Inc.
- 8.20.1 Company Overview
- 8.20.2 Key Executives
- 8.20.3 Company snapshot
- 8.20.4 Active Business Divisions
- 8.20.5 Product portfolio
- 8.20.6 Business performance
- 8.20.7 Major Strategic Initiatives and Developments
- 8.21 Janssen Pharmaceuticals
- 8.21.1 Company Overview
- 8.21.2 Key Executives
- 8.21.3 Company snapshot
- 8.21.4 Active Business Divisions
- 8.21.5 Product portfolio
- 8.21.6 Business performance
- 8.21.7 Major Strategic Initiatives and Developments
- 8.22 Inc.
- 8.22.1 Company Overview
- 8.22.2 Key Executives
- 8.22.3 Company snapshot
- 8.22.4 Active Business Divisions
- 8.22.5 Product portfolio
- 8.22.6 Business performance
- 8.22.7 Major Strategic Initiatives and Developments
9: Analyst Perspective and Conclusion
- 9.1 Concluding Recommendations and Analysis
- 9.2 Strategies for Market Potential
Scope of Report
| Aspects | Details |
|---|---|
By Type |
|
By End-User |
|
Report Licenses
Our Team

Frequently Asked Questions (FAQ):
What is the estimated market size of Multiple Myeloma Diagnostic in 2031?
+
-
What is the growth rate of Multiple Myeloma Diagnostic Market?
+
-
What are the latest trends influencing the Multiple Myeloma Diagnostic Market?
+
-
Who are the key players in the Multiple Myeloma Diagnostic Market?
+
-
How is the Multiple Myeloma Diagnostic } industry progressing in scaling its end-use implementations?
+
-
What product types are analyzed in the Multiple Myeloma Diagnostic Market Study?
+
-
What geographic breakdown is available in Global Multiple Myeloma Diagnostic Market Study?
+
-
Which region holds the second position by market share in the Multiple Myeloma Diagnostic market?
+
-
How are the key players in the Multiple Myeloma Diagnostic market targeting growth in the future?
+
-
What are the opportunities for new entrants in the Multiple Myeloma Diagnostic market?
+
-

